There is a lot of confusion between these two terms, probably because they both carry the name distillation. Many people confuse them. They are different terms with different meanings. They both perform different functions and have different features.
Distillation alone is the process of separating the mixture to get its fractions or parts. Chemical compounds boil to a temperature where one or more fractions of the mixture will evaporate in the process.
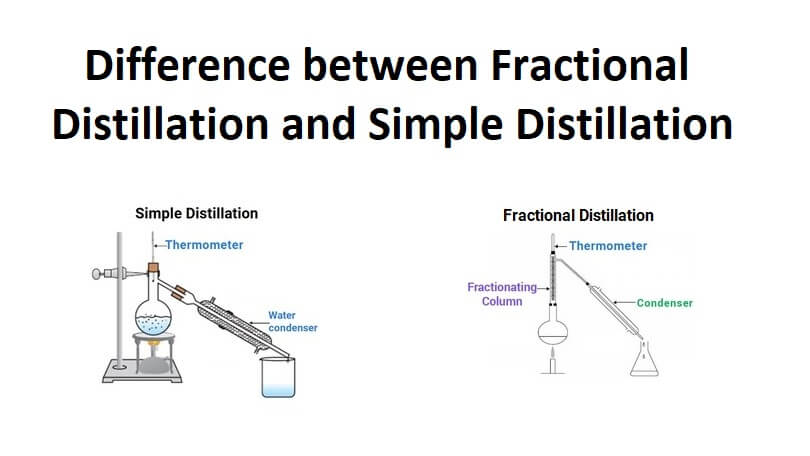
Simple distillation is the process of splitting up two liquids boiling at different temperatures. It is a method used to part liquids, which have a fifty degrees boiling temperature difference. Water distillation is a process of simple distillation.
Fractional Distillation Vs. Simple Distillation
The main difference between fractional and simple distillation is that the fraction distillation deals with chemicals with a boiling temperature close to each other. Simple distillation on the other hand deals with chemicals with a fifty degrees temperature difference.
A single distillation cycle is enough to separate the mixtures in the simple distillation process. Fractional distillation will require at least two or more distillation cycles.
Comparison between Fractional Distillation and Simple Distillation
- Fractional distillation helps to separate the mixture into its parts. Simple distillation on the other hand helps to separate two liquids with different boiling temperatures.
- Fractional distillation is able to separate a mixture whose boiling points are close to each other. Simple distillation separates liquids with fifty degrees temperature difference.
- Fractional distillation is used to refine crude while simple distillation is used to purify seawater.
- The apparatus used in fractional distillation is complex. It is used with a fractioning column. Simple distillation uses the same complex apparatus with fractionating column.
- Fractional distillation involves a repeat of the same process to get a pure solution while simple distillation gives the pure solution within the first attempt.
What is Fractional Distillation?
Fractional distillation is the process of separating mixed chemicals whose temperature degrees are less than forty degrees. It is a process that involves several simple distillation processes using one apparatus.
The chemical is packed in a metal wire, glass beads, or metal ribbon. This helps to give an extra condensing surface to the process of distillation. Fractional distillation is a process that ensures the condensation reaches its condenser within the shortest possible time.
An example of fractional distillation is the crude oil that is split up into several components. The liquid mixture in fractional distillation is used in a fractionating column because it has a similar boiling point.
Fractional distillation requires patience. This is because you need to repeat the same process over and again, to get the desired pure component. Unlike simple distillation that needs just one try, fractional distillation will require several attempts. Understand the exact components you need. For instance, the process of fractional distillation is best with crude oil.
The gas created in the packing material being warmed up by the hot gas obtains the pure component.
What is Simple Distillation?
This is the method of breaking down two components that have different boiling points. The liquids with at least a fifty degrees boiling point difference can be split up using the simple distillation method. The liquid to be distilled is put under heat and the condensation formed will be the richest or purest in the mixture.
Small compounds are warmed up and then condensed under a relatively low temperature ranging between two and three degrees Celsius. Taking a good look at the distillation flask reveals the split-up compound.
Once the machine temperature starts changing, it is an indication that the pure compound is no longer being distilled. The temperature increases until the next compound with the lowest boiling point come close.
The distillate fraction becomes the compound that gets warm at the second-lowest temperature. The process goes on and on until the original mixture is set apart. The simple distillation process is best for liquids that have different boiling points.
Difference Between Fractional Distillation and Simple Distillation
- Both processes involve the breaking down of different liquid components under different temperatures to get a pure substance.
- If the two liquids being broken down have temperatures that are close to each other, they are broken down by fractional distillation. Simple distillation breaks down liquids whose boiling points are at least fifty degrees apart.
- Complex equipment with a fractionating column is used for fractional distillation processes while the simple distillation process uses simple equipment with a fractionating column.
- In order to get the pure component in fractional distillation, the process has to be repeated many times. In simple distillation, however, the process is only done once and gives the pure component outcome.
- You cannot split up the solvent-solute in fractional distillation but the same can be done in the process of simple distillation.
- The process of fractional distillation is used to refine components like crude oil while simple distillation is used to purify water.
- Fractional distillation and simple distillation are two processes used in science and chemistry subjects. The purpose is to get components that are pure at the end of both experiments. There is additional equipment required in the fractional distillation process which is called the fractionating column. The simple distillation process will not need additional equipment, because the process is only done once.
Conclusion
In conclusion, we can see that explaining both fractional distillation and simple distillation brings out their many differences. While both are processes of separating components or breaking them down to get a single pure solution, many processes are involved therein. Components have to have the same boiling temperatures in fractional distillation while they need to be at least fifty degrees apart in simple distillation.
Chemical processes require time and understanding, in order to get the desired results. For instance, it is important to understand that you cannot use liquids of the same temperature while using the simple distillation process. You also need to use liquids with the same temperatures while using fractional distillation. Understanding these simple details will give the desired results.