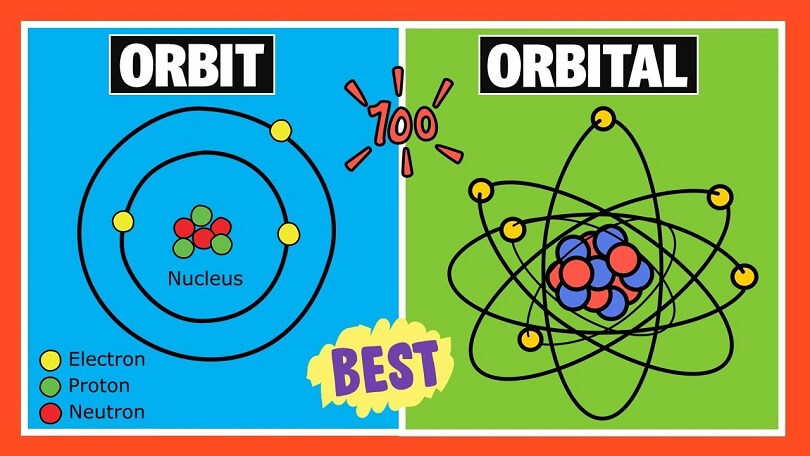
People who are unfamiliar with atomic theory often confuse the words ‘Orbit’ and ‘Orbital’, believing them to be synonymous. This, however, is not the case at all. Despite their similarities, both of these phrases are quite distinct from one another in a variety of ways.
Orbit Vs. Orbital
The primary distinction between an orbit and an orbital is that an orbit is regarded to be a clear route along which electrons rotate, but an orbital is thought to be a region that is unknown, and the odds of finding an electron here are at their highest. Any plane or two-dimensional region is considered to be an orbit. An orbital, on the other hand, depicts a three-dimensional region in which the likelihood of finding an electron is greatest.
The electrons in an atom may be thought of as planets in our solar system, with the nucleus representing the sun in this analogy. These electrons have a natural tendency to circle around a route known as an orbit. Even when electrons circle around their orbit, they continue to obey all of the same laws of motion that apply to planets as they revolve around the sun.
According to Heisenberg’s uncertainty principle, it is impossible to pinpoint the exact location of an electron with precision. When it comes to describing the location of an electron inside an atom, it is necessary to use the idea of an orbital.
Aspects like speed, direction, and location of an electron, however, are not specified by orbital. However, it provides an accurate estimate of the location of an electron.
What is Orbit?
According to the definition of orbit, an electron’s orbit is a predetermined route that is circular in form, on which it revolves owing to a magnetic pull experienced by the electron as it approaches the positively charged nucleus. According to Bohr’s atomic theory, this is true.
In addition, Bohr’s model predicts that the first shell of an atom will only contain two electrons. Later on, however, Bohr’s model was shown to be inadequate.
The orbital model is the most frequently recognized model at the current moment since it expresses the notion of the orbital. You must be familiar with the solar system in order to comprehend the concept of an orbit.
Consider the following: the planets in the solar system represent electrons, which are revolving around the sun, which represents a nucleus in this case.
While revolving around the nucleus, electrons follow Newton’s laws of motion, just as the planets do when they orbit the sun. An orbit, in contrast to an orbital, is just a two-dimensional journey through space. A single electron can occupy an orbit that can hold up to 2n2 electrons.
Following Heisenberg’s uncertainty principle, one cannot know the precise location of an electron with accuracy.
The idea of orbital comes into play at this point.
What is Orbital?
In accordance with the Heisenberg principle, it is impossible to determine the exact location of an electron in space or time. This is the first time we’ve heard of the orbital.
An orbital is a speculative region that indicates the area where there is the greatest likelihood of finding an electron. The orbital is represented as the three-dimensional region around the nucleus.
Orbital is capable of forming a variety of forms. Orbitals are commonly divided into four types: s, p, d, and f. S is the simplest kind of orbital. The s orbital has a maximum capacity of 2 electrons, but the p orbital can store up to 6, the d orbital can hold 10, and the f orbital can contain up to 16 electrons.
The likelihood of finding an electron in the three-dimensional area of an atom is relatively high, around 95 percent, in this region.
Because orbitals are directed, it is quite simple to identify the form of molecules using them. The Heisenberg principle plays a significant role in the operation of the orbital.
Difference Between Orbit and Orbital
- One definition of the orbit is a route on which an electron spins that is fixed in time. In contrast, an orbital is a three-dimensional route in which the probability of encountering an electron is relatively high (see Figure
- An orbital, on the other hand, does not claim to show the specific location of an electron inside an atom, but an orbital does not claim to depict the exact position of electrons.
- Electrons are discovered to be moving in an orbit in a two-dimensional or planar manner. Electrons, on the other hand, travel in an orbital pattern around the nucleus in three-dimensional space.
- Because orbits claim to be able to reveal the precise location of electrons, they are incompatible with Heisenberg’s Uncertainty Principle.
- The location of an electron in an orbital is not specified, and it might be anywhere in that three-dimensional region. As a result, it is consistent with the Heisenberg principle.
- Except for s orbital, none of the orbits exhibits any directed features, however, in the case of orbital, directional characteristics may be seen, with the exception of s orbital
Conclusion
Orbit and orbital are two separate phrases that are both connected to atoms. Orbit and orbital are both used to describe the same thing. Within an atom, the notion of an orbit is identical to the concept of planets circling around the sun within the solar system.
The motion of the electrons in orbit is perfectly consistent with Newton’s equations of motion. They do not, on the other hand, conform to Heisenberg’s Uncertainty Principle.
When we talk about orbitals, we’re talking about the 3D area surrounding the nucleus in which the likelihood of finding an electron is relatively high (see Figure 1). It adheres to the Uncertainty Principle established by Heisenberg in the letter.
According to Heisenberg’s Uncertainty Principle, there is no way to precisely predict the movement, speed, and direction of an electron inside an atom without using a quantum mechanical simulation.
Only a rudimentary depiction of where an electron may be found inside an atom can be obtained from the experiment.